Lavoisier
When chemistry was born, as an exact science, the world was in a state of all things at once, or in fast sequence forward: extraordinary grace at a time of the Enlightenment, sprouting bloody revolutions, and artistic or scientific fervor. Without this, according to my perhaps warped thinking: no present, no DNA guys, no CRISPR ladies! We visit here a father figure of heat and one of the chemical sciences, the latter introducing math and conceptual thinking to chemical reactions. They did away with much of myth and alchemy. So, going back in time, that’s what we will find in a massive way. Both were born around the time of Mozart’s birth in 1756 and I voice a tenuous suggestion that a pattern-rich and mathematically complex music will have sprouted on other planets also hand in hand with the advent there of mathematical science. All three cracked aspects of the nature of the world and are here tethered to each other. I begin with the artist who immediately gives a flavor of the times.
𓆤
Mozart’s world (1756-1791):
I would long to have a discussion with Mozart about anything. I find him charming from the documents that reached us and the literature it spawned. Well, perhaps not including everything; wouldn’t science be difficult after all? Religion would be deep and easy to talk about for a man who prepared for his death practically every day of his adult life with devotion to his God. But come to think of, wouldn’t it be bewildering to talk with Mozart about the structure of the world? You would meet under candle-light, one presumes, and ask what this is, this flame? It’s phlogiston escaping, Mozart may say under giggles. It’s a magical element, you know, it all is magic. You can see it: the candle disappears as phlogiston; no, that’s not it, you can’t see phlogiston of course. It’s what you can’t see, see, that’s hidden in the fire. And what is the heat? Well, that is also an element, you know, like fire, water and air and such things. I have it in my operas. It’s all magic! It’s a flute, a fluke.
Mozart could have known more, but almost certainly didn’t, not in detail that is. He could have known more because some of his contemporaries, especially in Paris were creating the know-how during his time. He would have picked up the atmosphere of mathematical reason by osmosis perhaps, even as it didn’t penetrate him to the extent of shedding all superstition. Mozart was afraid of ghosts, which made him terrified of the stranger who kept nagging him to finish the requiem; Mozart thinking that he was writing music for his own funeral. The undertow of unreason of an age past was still pulling at him. There was enlightened Lavoisier in 1777 already understanding revolutionary things about nature! But Mozart created his own understanding. I don’t think that without it, the sailing to our world would have been the same either. He gave many of us sense and motivation in his elaborate mathematically but foremost emotionally sounding worlds. Ask Einstein.
Mozart was twice in Paris: in 1763/64 as a seven-year-old wunderkind with his sister and father. And in 1777-1779 he was there as a 23-year-old (at the end of his stay). The later trip was with his mother to search for employment. The trip was a deep disappointment and his mother died on, and probably of it. Yet Wolfgang Amadeus took the occasion to conceive of the Sinfonia Concertante for Violin, Viola, and Orchestra, K364. It contains the know-how of how to deal with sorrow and how to remember joy and love. It is one of my favorite pieces of art of any.
Yes, Mozart could have met Lavoisier on the second trip, with the later as a 36-year-old tax collector entrepreneur, channeling his profits into his research. But the two were in different circles, where perhaps Lavoisier had heard of Mozart but perhaps not Mozart of Lavoisier. But who knows? Lavoisier was also a showman and perhaps Mozart was in the crowd for the entertainment of the big solar burning glass.
Mozart departed too early from Paris, but Lavoisier may have been privy to the preparations of gaudy historical balloon flights for the near future (1783). There were the Montgolfier brothers with their canvas hot air balloons, fancifully decorated in the emblems of the time, and voluminous and expansive (with a sheep, a rooster, and a duck aloft as first passengers and supposedly rising under “Montgolfier gas” as hot air). There was also, for later in the same year, the hydrogen balloon of Charles. It was much smaller, yet could fly much higher and had a silvery shine due to its mylar to keep the gas in. It looked uncanny to the farmers when it came down and they pierced it to pieces with their pitchforks. Superstition may have left Paris in the learned circles by then but not on the country. Yet the mylar model was a winner and supplied Charles with valuable meteorological info.
Mature if still young, Mozart found ways to weave old rhythms, melodies, and harmonies such that souls can resonate. Old cultural lines of happiness and sorrows, practiced from times immemorial on, mix to affirm humanity and motivate the living. Mozart was cracking open aspects of our common emotional world. And later generations can keep finding sustenance in this knowledge. The music can be bewilderingly complex, mathematically stimulating, say in the Jupiter symphony, like endless patterns of the world turning. The forms of the female of the human species, some of the Italian Vespa scooters or Delonghi heaters, a cello or Mozartian music are the most appealing for me.
There is the Mozart effect that supposedly showed a positive effect on test taking through some intellectual preparation in the hearing. Some years ago, it was also a fashion, or still is, to expose the infant, or the embryo already, to Mozart, intrauterine Mozart so to speak. This was to enhance the unfolding of the brain. To make Sapiens a little more sapient. To ensure that those brains are forming the maximum number of synapses. The hope was and is that children would reach their fullest potential and avoid drudgery, or even occasional urges for criminality. And that eventually they would comprehend Dalton’s stoichiometries and so be accepted to Medical School. That they would grow up to be financially independent and live lives of nothing worse than common despair and unhappiness, or best, become thriving money doctors filled with dedication to their calling.
𓆤
Antoine Lavoisier “of chemistry” (1743-1794):
It is generally accepted that Lavoisier's great accomplishments in chemistry stem largely from his changing the science from a qualitative to a quantitative one. Perhaps the most important novelty of Lavoisier’ study was the meticulously determined mass balance. It did away with phlogiston and introduced concepts of conservation of mass. This new thinking led to scientific Concept-Restructuring and chemistry taking its place in earnest amongst the sciences. In our time, the concept of phlogiston now looks to us as something grotesque and bizarre, like fascism.
Lavoisier’s entry into oxygen started in 1777 (chemistry being born in an equally memorable year to physics’ 1666 with Newton’s apple) when he published a new theory of combustion. For this he needed his “new” gas, which he named ‘oxygen’, meaning ‘acid-producer’, because he mistakenly thought it to be an essential component of all acids (The nature of acids was not clarified until Sir Humphry Davy’s work in 1812). Lavoisier’s matter balancing chemistry appeared just before 1800 (Traité élémentaire de chimie or Elementary Treatise on Chemistry was published in 1789). It was to become the founding of chemistry, as we know it.
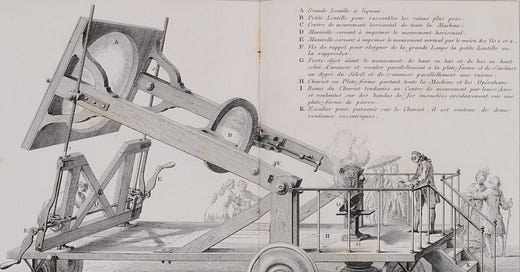
One of his hallmark experiments was to follow the decomposition and reformation of mercury oxide, say 100g. The gas, liberated on heating the oxide, is oxygen (7g), while the red oxide decomposes into the whimsical mobile metal mercury (93g). The reaction is reversible with temperature. The balance of masses stays constant. Similar reactions can be run with mercury sulfide, the red cinnabar, and here a decomposition product is the yellow sulfur contributing to the magic of color in chemistry. The reactions had all the trappings of age-old alchemical ones; but now they were amongst the first really understood ones. Lavoisier delighted in expensive equipment with large burning glasses, to get the heat. He attended some of those publicly with Mozartian élan. And well he should, as he was to become a match for Mozart, if in his field.
He had built his laboratory in Paris in a gunpowder arsenal on the right bank of the Seine River. For a time, he also maintained a private apartment close by. His much younger wife assisted him at every turn. She was an especially adept draftswoman, supplying us with many vivid depictions of scientific equipment and laboratory life.
Amongst the main equipment was a vessel with a beaked cap or head, formerly often employed in distilling, such as derivatives of the Egyptian Alembic. It has been a symbol for the Egyptian art: Al Chymia. In fact, these reactions, say on mercury compounds, had been known for over a thousand years. What was new, was the analytical and experimental attitudes brought to their execution and interpretations. Measurement quantification became the hallmarks of their approach. Lavoisier produced a balance accurate to less than one one-thousandth of a gram; and he co-invented the calorimeter to measure small temperature changes in chemical reactions.
Lavoisier basically reinvented chemical nomenclature with coinages like “carbonate,” “nitrate,” and “sulfate”. He did this even though many felt them “harsh and barbaric words that shock the ear and are not at all in the spirit of the French language.” For him clear language and signs enabled greater analytical creativity. He showed that water is formally produced by the explosive combustion of “dephlogisticated air” and “flammable air”. He gave these reactants now new names as “hydrogen” and “oxygen”, replacing the evocative terms of an earlier time. Likewise, and perhaps deplorably, the butter of bismuth also became a prosaic oxide of bismuth.
The driving force for Lavoisier’s work appears to have been entrepreneurial, as it was with many of his contemporaries. Many showed themselves to the world with their great experiments and wanted to profit from it. Lavoisier wanted to set up his France, not yet more but already all of it, as running efficiently and based on exploiting nature. Before him there was perhaps a somewhat more spiritual note to discovery in searching for the nature of God’s world (Newton). These people were mostly deeply religious. After Lavoisier, scientists were driven by a more abstract desire to improve the lot of humanity, all of it and becoming more academic and not self-serving (Roentgen, Curie). Perhaps the most basic common driving force of these founding generations for science was curiosity. It had dawned on people that one could find out. And this curiosity was satisfying by itself. Presently, the driving forces in science are a broad mix with academic and entrepreneurial amongst others, with perhaps a slight imbalance towards the latter for optimal innovation.
𓆤
The French Revolution’s bloody work:
While the French Revolution started doing its bloody work, Lavoisier initially continued with his explosive one of “blast gas”. In 1789, Lavoisier, like many other philosophically minded administrators, saw initially the revolution as an opportunity to rationalize the nation’s economy. On a related level, the revolution had introduced the metric system. This meant the need for logarithms to be recalculated on a base of 10 system. It amounted to one of the great calculating projects of the late 18th century. People not trained as mathematicians accomplished this gargantuan task by hand in record time. Anecdotes have it that many of them were unemployed people like cooks and hairdressers. They may previously have served in noble households that were dissolved when their masters were guillotined or fled. The tables were much used for nearly two centuries until recently when calculators took their place. But before that the bland looking values would have intriguing stories to tell from these unstable times.
Even as events turned bloody, Lavoisier, perhaps overestimating the authority of science, continued to advise revolutionary governments and neither he nor his wife fled abroad. Lavoisier continued to argue that the Academy of Sciences should be saved because its members were loyal and indispensable.
Lavoisier wrongly didn’t think he had to lose his head over this, even as developments turned ominous. Basically, the revolution was primarily spurred on by the Enlightenment’s abstract theories of equality. These theories originated in Paris’s fashionable literary salons; lawyers and journalists spread them; and Lavoisier felt as part of this. In this light, the Revolution’s central paradox will stay a question for the ages. How could hundreds of thousands of French citizens be slaughtered in the name of “liberté, égalité, fraternité? How could the Revolution degenerate just when clear headed science was born. What was the sense of the Nantes mass drowning (“vertical deportations”)?
For some time, developments hang in the balance. Would one avoid violence? Would those who could be expected to promulgate the point come to influence? Indications for the bloodshed to come arrived when Camille Desmoulins and Jean-Paul Marat called for revolutionary violence. By 1790, one concerned observer noted the “wild and dangerous effervescence” of the Jacobins.
The Terror began in 1792 when the Convention suspended the constitution and adopted the Law of Suspects. This permitted expedited trials and executions of counterrevolutionaries. Robespierre, appointed to the Committee for Public Safety in 1793, became the de facto dictator of France. He said: “The foundations of a popular government in revolution are virtue and terror, terror without virtue is disastrous and virtue without terror is powerless.” Thousands of Parisian executions later, Robespierre was executed himself on July 28, 1794, and the fervor began to abate.
But just before that abating, Lavoisier’s affiliation with tax collection was to cost him his head after all. He and his father-in-law and others were hauled off and executed on May 8, 1794. There was no need for savants a judge had pronounced.
𓆤
Survival of Lavoisier’s work:
Yet Lavoisier’s work survived. After his insights, recipes were now specific on how to name chemicals in a functional way rather than by mythical names. A quantitative chemical language had been developed that only needed augmentation by the actual atomic theory of Dalton. In a practical sense, Lavoisier initiated chemical science, if yet without biology. It was begun in ancient Alexandria as semiquantitative physic, and had a renaissance, paradoxically in the early Baroque with Galileo, Kepler, or Newton. The time of Mozart and the big revolutions mark a plausible date for the completion of science’s birth. In Mozart’s music it became an explosion of invention within a structured geometry, and in some sense, Lavoisier’s was a similar invention. Lavoisier was even close to have understood the music of what he called the “imponderable matter of heat”.
Heat, at the time, was considered amongst others a mythical fluid, called caloric, that surrounded all atomic matter in its nooks and crannies. It was deemed weightless and capable of being drained away from reactions that produced it. When a gas is compressed, the particles of caloric supposedly were squeezed out as they repelled each other at close distance. Heat of friction was accordingly due to the rubbing off of caloric through the frictional motion. None of which nourished any understanding of what happens when this rubbing would be done to exhaustion. In reality, this rubbing is never losing effectiveness because nothing is lost, but rather heat or molecular motion is gained and with it even some minute mass in Einstein’s mass-energy equivalence. Yet, most people during the first half of the 19th century would go along with these questionable ideas (the idea is not in all ways non-functioning, as in the energy mass equivalence there is a mass to heat, and this mass can remind of caloric).
𓆤
Count Rumford, alias Benjamin Thompson “of heat” (1753-1814):
Our different present day understanding of heat as molecular motion is originally due to an enterprising and colorful American. He, Benjamin Thompson, was born 1753 in Massachusetts but his fate made him travel to places where science was at its most active at the time: London first, then Munich, and then Paris, where he died in 1814.
In Munich he was employed supervising the drilling of cannon shafts. For this purpose, horses would move a grinder around the shaft having its inner cylinder polished. The shafts were placed in water, to carry away the heat of friction. Thompson observed that the water would come to a boil after equal time intervals and there was never a limit of heat released, as one would have to have assumed with the concept of caloric. So, he argued that heat corresponded to the only thing that was continuously, and seemingly ad infinitum introduced: namely motion. And this motion was transferred to the atomic level as molecular motion. Heat is then nothing but molecular motion, he asserted around 1788-1790 in Bavaria. Not far away, in Vienna, Mozart was to enter his last year, most likely blissfully untouched by cannon boring but filled with the cosmos of his requiem.
𓆤
Strange heat concepts:
Although correct by today’s understanding, Thompson’s ideas, for many reasons, amongst them his character, didn’t carry the day. They had to wait for the second half of the 19th century with names like Joule, Helmholtz, Gibbs, Boltzmann etc. Those were the thermodynamicists who experimented with heat transfer, quantifying it, and seeing that it determines the arrow of time or the direction the world goes. But they still wouldn’t say what it is in the sense of what molecular motion means.
Later, quantum mechanics dealt with molecular motion as coming in measured parcels of phonons. The pendulum-motions of molecular oscillations are not free to swing as they please; they are confined to specific values. Just think how strange this is that motion is not gradually increasing or decreasing but making jerky jumps.
You excite those motions by pouring heat into say a solid. Temperature then rises but, in a way, depending on the heat capacity of the solid. Temperature can rise little or much with the same heat input, depending on material and the molecular motion it allows. Temperature then is a measure of the ability of a substance, or more generally of any physical system, to transfer heat energy to another physical system. It is a state of excitement, an intensive property, by comparison with the extensive heat. And temperature controls the direction events take; heat will always flow from the hot to the cold. So, where does our heat go to? Well, to outer space where it is 3K cold. And is this the end? No, a Black Hole can be colder yet. More of this later.
And to return for further thought: as heat is molecular motion, energy then is always motion or its potential, say in falling from a tabletop. And motion increases the mass in the Einstein sense, as though you had thrown matter dust on it in a lopsided way (for matter to increase to the speed of light the mass would increase to infinity).
So, temperature measures the transport potential of the mass equivalent of the energy of motion. We can call this mass equivalent “energy or mass dust” of quantum kernels, settling between oscillating atoms (the dust picture is used in textbooks, say for the probability of finding an electron in the Schroedinger equation). Perhaps one can imagine kernels in the form of strings to add to the propelling like flagella attached to one side of the mass? It is not a common way of seeing things but goes some distance concerning Feynman’s dictum that we don’t know what energy is. It is an attempt at pictorial representation.
The direction of flow of “energy dust” is then governed by temperature. Even today we think that heat is energy that can settle between atoms. The question is more important than would meet the eye at first sight because heat is the desired product in the basic drives, the final aim of nature. Energy then appears to be basic stuff, something like quantum kernels. We will keep returning to these enigmas. But this the time to remember the boring of Bavarian cannons! Startling it was, nothing boring, this boring! The cannons’ boiling water coolers set the Beeline in motion to basic understanding.
One reason Thompson was not initially followed on the principle of “heat as molecular motion”, may have had to do with his questionable personality. This discounting of achievement due to personality is rare in science nowadays, although it appears to work some, as sometimes perhaps on the level of grant income. Thompson was from some indications largely without principles, toadying sweetly to his superiors, being acidic to his peers and outright caustic to his subordinates. With this mix of contradictory chemical metaphors for character he was to make a lot of enemies and in the process had to change his address repeatedly. His enormous energy and self-starting enterprises made him achieve remarkably nevertheless, especially in the field of organization and invention, where he can be compared with B Franklin and TA Edison. What he lost in stability he apparently gained in getting to know new places and people and opportunities.
𓆤
Modest beginnings and a shady career:
As a son of a large farm family Thompson had a modest beginning as an apprentice and schoolteacher in Concord, then called Rumford. These callings didn’t quite pan out. Yet here he made a first of his typical steps upward by marrying a young widow of means. The resulting connections made him of interest to the British Royal Governor. And so, his career started with being a spy for the British, informing of armaments and supplies of the Colonial Militia and Minuteman. And that’s where all his troubles and excitements started.
While charged by the patriots with being “unfriendly towards the cause of freedom”, his cleverness made sure that nothing could be proven in the end. Yet just before Christmas 1774 the patriots came with feathers and tar after him after all and he had to ride for Boston in the dark of night, leaving wife and baby daughter Sarah behind, never to see them again. From then on, they were staying unsupported by him, yet not having to support him either.
He continued spying, now for Massachusetts, having lost some credibility through his previous adventures though. So, when the British left Boston, in a huff in 1776, he had the good sense to go with them to London. There he found employment as an expert on the Revolutionary War for all of seven years, making important scientific advances on the side. Yet, when he was suspected to slip information to the French, he escaped to Munich as military advisor. Would he now sell information of the Bavarian army back to England? You bet.
𓆤
Doing a lot of good in Munich:
At the same time, he improved the Bavarian state and army, not too dissimilar to the role of Lavoisier in France, although in the end with more luck. They were both sort of independent contractors who collected a fixed amount from taxes for their mission, keeping a balance for their own profit by cutting expenses. Colonel Thompson so experimented with cannon boring or the preparation of food or clothing. And with these tasks began his ascent to greatness for which he was honored by promotion to a count of the Holy Roman Empire as Count Rumford, he so rhapsodizing his place of birth, even though a count of counts there in the US would have produced about zero.
When he asked for manufacture of clothing to his specification, and was not able to obtain it, he enlisted an assortment of beggars from the street. With them he had the military workhouses transformed into factories, producing what he required. In return he provided his new workers with room and board and established free schools for children. For the poor he devised his nourishing Rumford soups. For enjoyment he established one of the largest city parks anywhere, the English Gardens.
He introduced potatoes for nutrition although the people first didn’t like them, even as eventually they wouldn’t kick the habit. He praised coffee for stimulating thinking. The Turks had brought coffee to the gates of Vienna in 1683, and Count Rumford invented for it a drip and a percolating coffee pot. Other practical inventions ranged from the kitchen range, the baking oven as well as metallurgical furnaces. The Rumford fireplace conquered London. He restricted the chimney opening to increase the up draft. For practical purposes he modified fireplaces by inserting bricks into the hearth to streamline the flow and avoid its wallowing into the room of exhaust and so suffocating its inhabitants. In fact, the management of heat was a recurring theme in his work, as he also became known for the invention of thermal underwear.
𓆤
Paris:
In 1795 Thompson’s health had declined due to his hectic schedule, and he had made numerous enemies so that he felt it time to move on. This time it was to Paris during the beginning of the Napoleonic wars. Paris was the place where Maximilien Robespierre cried in 1792, as he prepared the French for a reign of terror: “Citizens did you want a revolution without revolution?” Robespierre had been executed and shortly thereafter the times were changing from ones where the Revolution would pronounce proudly that it had no need for savants. Now they were in need again. Yet surprisingly after 1799, Thompson returned part time to England, a rather enterprising move at this bellicose time between France and Britain but fully in keeping with his character, bent on exploiting espionage opportunities of war wherever there opened the slightest opportunity to streamline the flow. There he became cofounder of the Royal Institution of Great Britain. Sir Humphry Davy became the first lecturer; and his assistant, Michael Faraday established the Institution as a premier research laboratory, becoming perhaps the most far-reaching experimentalist in history, and another true canary in science’s gold mine.
In 1804, back in Paris for Napoleon’s coronation to emperor, Thompson married the widow of Antoine Lavoisier, his American wife leaving notice of having died by then. But Thompson separated from his second wife after 3 years, perhaps to continue his scientific work in Paris until his death in 1814? One suspects that divorce was more likely because he was a difficult character. This was also the time of the first abdication of Napoleon and the new beginning of the Congress of Vienna. Upon his death, his daughter from his first marriage inherited his useless title as Countess Sarah Rumford but no money. He bequeathed all his possessions to Boston’s Harvard University, which has become today one of the leading research places, second only in citations to the University of California.
In Thompson we see a new polyglot scientist, engaged heavily with government, travel, and occupied with its practical large-scale enterprises. Europe at that time is open for talent and willing to employ it beyond borders, while presently the emigration is still more towards the US. As an example, in Munich as the Reichsgraf, Thompson was the second most influential person. We see also a conflicted person. On one side there were his accomplishments of doing good to the poor, his Rumford soups. We note his improvements in nutrition, heating, clothing, or housing, which made noticeable changes in the life of many Europeans. On the other side there remained his abrasive character with his peers and subordinates. Nobody wanted to credit him upon his death, and contrary to say Lavoisier, Dalton, or Franklin, he was quickly forgotten. He was a high-IQ ping pong ball trying out the limits until off table. But he is worth to be rediscovered and one can be looking forward to, say, a movie about the brilliant plotter and his updrafts. In a modern analogy he behaved like a wide swerving car. His tires would be screeching and scrape the curb and constantly popping off their hubcaps. But he would be covering distance if hitting many undeserving bystanders.
The question of heat was far from over. To its continuing intrigue added a series of experiments by Marc-Auguste Pictet in the late 18th century that demonstrated quite puzzlingly that cold, like heat, could be reflected from a mirror. Apparently, it has a radiation component. Temperature scales were developed, a multitude of them, and this period of scale confusion has not ended and still does damage to our economy.
Presently we know how to make measurements of heat’s differentials, we can measure its effect on thermometers and have at hand a self-contained system within the concept of molecular motion. But we really don’t know what heat or energy is on a more fundamental quantum granular basis, as Feynman pointed out.
𓆤
A note on catalysis:
In previous essays I have seen catalysis as one of the most important principles in the drive of the world. I have called us and other bionauts as catalysts for energy transformation. Here I want to give some of its history.
An important advance in understanding of reaction speed was made by Scottish chemist Elizabeth Fulhame in a 1794 book, based on her work on oxidation-reduction experiments. Her aim was to create fabric of metals such as gold, tin or silver. In her chemical hydrogen reduction reactions from metal salts, she noted that speed was influenced by the presence of trace water, which was though not consumed. This was the first clear case of catalysis. Count Rumford thought highly of her as "the ingenious and lively Mrs. Fulhame." Joseph Priestley encouraged her to publish her 14 years of research in 1793 after a meeting.
Berzelius in 1836 coined the name “catalysis”. He considered that besides chemical “Affinity” a new “Catalytic Force” could be active. Greek is the root of the word meaning down and loose (prefix cata appearing in catapult or cataract). Berzelius used the concept to describe reactions that are accelerated by substances that remain unchanged after the reaction. Johann Wolfgang Döbereiner spoke of contact action, leaving us Döbereiner's lamp based on hydrogen and a platinum sponge. It became a commercial success in the 1820s.
So, the lively Mrs. Fulhame gave us catalysis. This concept can rival entropy, with respect to the drives of the world, in that catalysis accelerates or selects amongst them. In our vision, say of Bio- or of Catanauts, as living cyber steered movable catalysts, we focus on digestion of a mature organism as the main catalyzed reaction (We neglect actual material uptake).
𓆤
Pictures of the times:
Mozart: the Lange portrait is probably the least adulterated one of the master. Lange had married the sister of Mozart’s wife, who was Mozart’s original love interest. Mozart appears to have taken it in stride and with his customary coarseness: “when the thing doesn’t like me then she can lick …” Mozart-1783-lange.jpg (467×600) (wikimedia.org)
Lavoisier: he used his profits from tax collecting for experiments that put a foundation under chemistry. File:Portrait of Antoine-Laurent Lavoisier and his wife.jpg - Wikimedia Commons; Œuvres de Lavoisier Paris, Imprimerie impériale, 1862-93 RGNb10341936.22.vol III.plate IX - Antoine Lavoisier - Wikipedia
Gas experiments: the paintings of Wright depicted scientific themes in the reverential manner formerly reserved for scenes of historical or religious significance. An Experiment on a Bird in an Air Pump by Joseph Wright of Derby, 1768 - An Experiment on a Bird in the Air Pump - Wikipedia
Rumford: is a character in a caricature linked in the last essay. Another one, this of his work with heat, is here: James Gillray: The Comforts of a Rumford Stove (james-gillray.org)
🐝🐝🐝 🐝🐝🐝 🐝🐝🐝 🐝🐝🐝
On our way back still further, we will now enter a scientifically much more austere landscape, if an adventurous one. In it we search for the handling of vacuums that will eventually catapult us through eons back into antiquity. Over a short timespan, art and music will stay at high level. Then myth and superstition will rise, and a general tendency for a freeze of all things savvy, until a brief reverse renaissance with Alexandria.
🐝🐝🐝 🐝🐝🐝 🐝🐝🐝 🐝🐝🐝